Article Today, New Delhi:
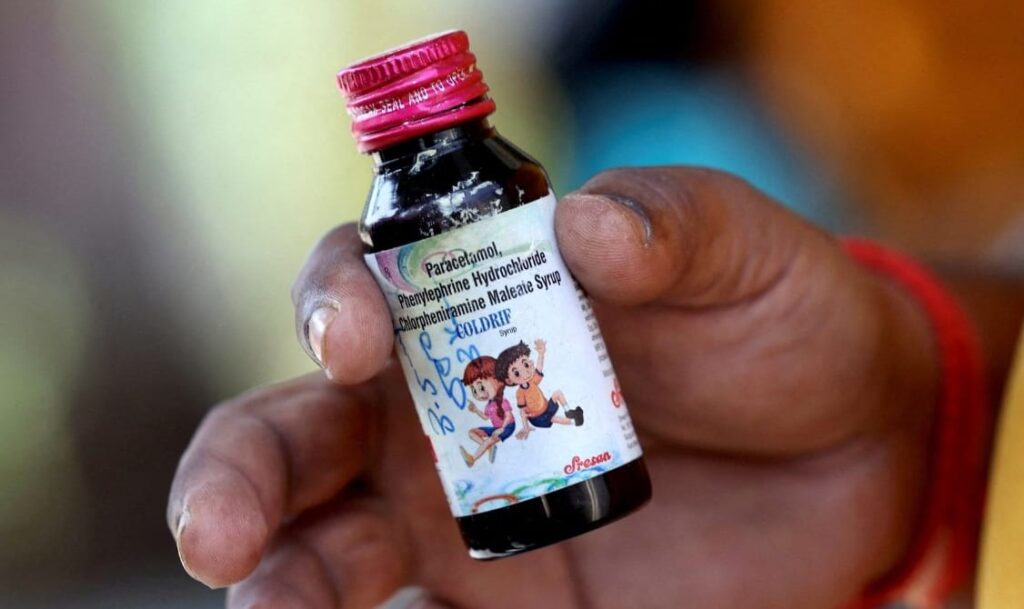
The recent discovery of dangerously high levels of diethylene glycol in an Indian-manufactured cough syrup has sparked outrage and raised serious concerns over the country’s drug regulatory system. At least 17 children, all below the age of five, have died after consuming the syrup, identified as Coldref. Tests revealed that the chemical concentration was 500 times higher than the permissible limit.
Toxic Substance Behind the Deaths
Diethylene glycol, a toxic industrial solvent, is known to cause severe kidney damage, neurological failure, and death. Experts warn that even small quantities can be fatal, making the 500-fold excess level a matter of grave public health concern. The findings have reignited debate over pharmaceutical safety and accountability in India.

More Brands Found Contaminated
In a disturbing development, two additional cough syrup brands — Respifresh and Relife — have been banned in several states including Telangana and Gujarat. Preliminary tests confirmed the presence of the same poisonous chemical. Authorities have issued urgent alerts, while investigations revealed that one brand was distributed locally and others sold across multiple states. Despite these findings, national drug regulators have been criticised for delayed action.
WHO Seeks Clarification
The World Health Organization (WHO) has asked the Indian government for an immediate explanation regarding the safety and export of these formulations. The incident has again dented India’s reputation as a global pharmaceutical hub. WHO officials are reportedly awaiting official confirmation before issuing a global alert against Coldref. The situation recalls past tragedies in Gambia, Uzbekistan, and Cameroon, where hundreds of children died after consuming Indian-manufactured cough syrups.
Regulatory Failure and Lapses
The Drug Controller General of India, Rajeev Raghuvanshi, admitted that the manufacturers failed to test raw materials and final products as required by law. This breach of protocol, experts say, exposes deep flaws in the quality control framework. Despite repeated warnings, inspections of substandard manufacturing units remain superficial and inconsistent.
Factory Abandoned After Tragedy
The plant operated by Shreeson Pharmaceuticals, identified as the producer of Coldref, has reportedly been shut down. Investigators found the premises abandoned, with unlabelled bottles, burnt stock, and strong chemical odours — stark evidence of regulatory neglect. Officials said the company’s owner is currently untraceable.
A Reputation at Risk
India’s pharmaceutical industry, valued at nearly USD 50 billion, faces mounting scrutiny. While it remains one of the world’s leading suppliers of affordable drugs, repeated safety scandals have eroded international confidence. Experts urge immediate reforms in manufacturing oversight and stricter enforcement of testing norms to prevent further loss of life.
Call for Accountability
The tragedy has once again exposed the cost of regulatory indifference. As investigations continue, health authorities and industry stakeholders face increasing pressure to restore credibility and ensure that the pursuit of profit does not come at the price of human lives.